Identify whether each set of quantum numbers is valid., a topic of paramount importance in quantum mechanics, unveils the intricate rules governing the behavior of electrons within atoms. By delving into the concept of quantum numbers, their significance, and the criteria for their validity, we embark on a journey that illuminates the very foundations of atomic and molecular physics.
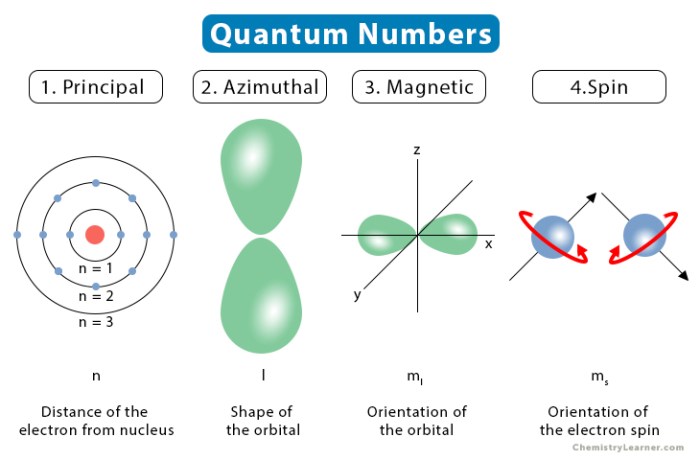
The four quantum numbers, namely n, l, ml, and ms, each carry distinct physical meanings and impose specific constraints on the possible values they can assume. Understanding these constraints is crucial for comprehending the behavior of electrons and predicting the properties of atoms and molecules.
Quantum Numbers and their Significance: Identify Whether Each Set Of Quantum Numbers Is Valid.
Quantum numbers are a set of four numbers that describe the state of an electron in an atom. They are essential for understanding the behavior of electrons and the properties of atoms and molecules.The four quantum numbers are:
- Principal quantum number (n): describes the energy level of the electron.
- Azimuthal quantum number (l): describes the shape of the electron orbital.
- Magnetic quantum number (ml): describes the orientation of the electron orbital in space.
- Spin quantum number (ms): describes the intrinsic spin of the electron.
Validity of Quantum Number Sets
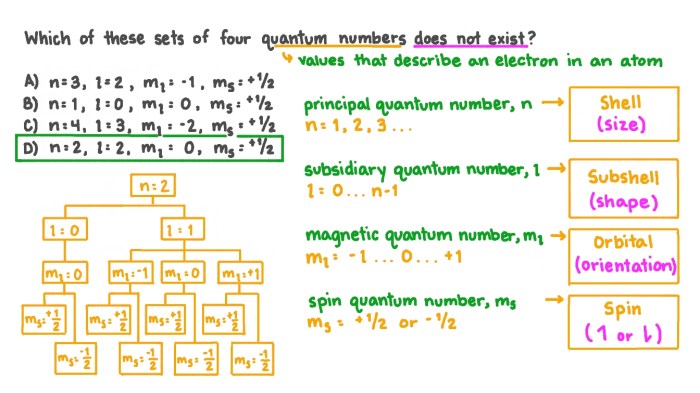
A set of quantum numbers is valid if it satisfies the following rules:
- n must be a positive integer (1, 2, 3, …).
- l can be any integer from 0 to n-1.
- ml can be any integer from -l to l.
- ms can be either +1/2 or -1/2.
Any set of quantum numbers that does not satisfy these rules is invalid.
Identifying Valid and Invalid Sets
Here are some examples of valid and invalid sets of quantum numbers:Valid sets:
- (n=2, l=1, ml=0, ms=+1/2)
- (n=3, l=2, ml=-1, ms=-1/2)
Invalid sets:
- (n=0, l=1, ml=0, ms=+1/2)
- (n=3, l=3, ml=2, ms=-1/2)
The first set in the valid examples satisfies all the rules: n is a positive integer, l is between 0 and n-1, ml is between
- l and l, and ms is either +1/2 or
- 1/2. The first set in the invalid examples violates the rule that n must be a positive integer. The second set in the invalid examples violates the rule that l can only be between 0 and n-1.
| Set | Validity | Reason |
|---|---|---|
| (n=2, l=1, ml=0, ms=+1/2) | Valid | Satisfies all rules |
| (n=3, l=2, ml=-1, ms=-1/2) | Valid | Satisfies all rules |
| (n=0, l=1, ml=0, ms=+1/2) | Invalid | n must be a positive integer |
| (n=3, l=3, ml=2, ms=-1/2) | Invalid | l can only be between 0 and n-1 |
Applications and Implications, Identify whether each set of quantum numbers is valid.
Identifying valid quantum number sets is important for understanding the properties of atoms and molecules. Valid quantum number sets can be used to predict the energy levels of electrons, the shapes of electron orbitals, and the magnetic properties of atoms and molecules.
Invalid quantum number sets, on the other hand, cannot be used to make any predictions about the properties of atoms and molecules.
Essential Questionnaire
What is the significance of quantum numbers?
Quantum numbers are essential for describing the state of an electron within an atom. They provide information about the electron’s energy, shape of its orbital, orientation in space, and spin.
What are the rules governing the possible values of quantum numbers?
The rules governing the possible values of quantum numbers arise from the wave-particle duality of electrons. These rules ensure that the electron’s wavefunction, which describes its behavior, is a valid solution to the Schrödinger equation.
How can we determine the validity of a set of quantum numbers?
The validity of a set of quantum numbers can be determined by checking if they satisfy the following criteria: n ≥ 1, 0 ≤ l ≤ n-1, -l ≤ ml ≤ l, and ms = ±1/2.