Introducing the intriguing question of does hydrogen have more electrons than uranium, we embark on an exploration that delves into the depths of atomic structure and electron configuration. Hydrogen, the lightest element, and uranium, a radioactive heavyweight, possess distinct electron counts that shape their chemical properties and practical applications.
In this comprehensive analysis, we will unveil the fascinating world of electrons, unraveling the mysteries behind their distribution and impact on the behavior of these two elements.
Element Overview
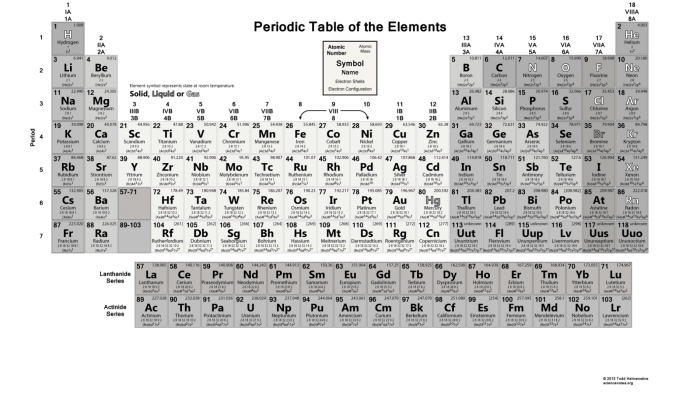
Hydrogen, with an atomic number of 1, is the lightest and most abundant element in the universe. Its electron configuration is 1s 1, meaning it has one electron in its first energy level. Uranium, on the other hand, is a heavy metal with an atomic number of 92. Its electron configuration is [Rn]5f 36d 17s 2, indicating that it has 92 electrons distributed across seven energy levels.
The atomic number of an element is equal to the number of protons in its nucleus. Since protons and electrons have equal but opposite charges, the number of electrons in an atom is also equal to the atomic number.
Electron Count Comparison
Based on their atomic numbers, hydrogen has only one electron, while uranium has 92 electrons. This significant difference in electron count is due to the difference in their atomic numbers.
Electron Configuration
The electron configuration of hydrogen is relatively simple, with its single electron occupying the 1s orbital. Uranium’s electron configuration, however, is more complex due to its numerous electrons. The 5f, 6d, and 7s orbitals are all partially filled, contributing to uranium’s unique chemical properties.
Chemical Properties
The difference in electron count between hydrogen and uranium has a significant impact on their chemical properties. Hydrogen is a highly reactive element that readily forms bonds with other elements. This is because its single electron can be easily shared or transferred to achieve a stable electron configuration.
Uranium, on the other hand, is less reactive due to its many electrons and filled outer orbitals.
Applications, Does hydrogen have more electrons than uranium
Hydrogen’s high reactivity makes it a valuable fuel source. It is used in fuel cells, rocket propellants, and other applications where a clean and efficient energy source is required. Uranium, on the other hand, is primarily used as a fuel in nuclear power plants.
Its ability to undergo nuclear fission releases vast amounts of energy, making it a powerful energy source.
Question & Answer Hub: Does Hydrogen Have More Electrons Than Uranium
Why does hydrogen have fewer electrons than uranium?
The number of electrons in an atom is determined by its atomic number, which is unique to each element. Hydrogen has an atomic number of 1, indicating it has one electron, while uranium has an atomic number of 92, giving it 92 electrons.
How does the difference in electron count affect the chemical properties of hydrogen and uranium?
Hydrogen’s single electron makes it highly reactive, readily forming bonds with other elements to achieve a stable electron configuration. Uranium, with its numerous electrons, is less reactive and tends to form stable compounds with other elements.